Discover the XDEMVY® Effect

XDEMVY Targets and Kills the Mites That Cause Demodex Blepharitis (DB)1


Can You See the Difference XDEMVY Has on DB?
Witness the real impact XDEMVY has on eyelids with DB for yourself! Try to successfully match up eyelids before treatment with XDEMVY to the same eyelids after treatment.
Real patients below with DB used XDEMVY twice daily, in each eye, for 6 weeks. Results may vary.
THE RULESBefore XDEMVY
After XDEMVY
Now that you’ve matched all the BEFORE and AFTER images successfully, submit your name and email to learn more about XDEMVY.
XDEMVY is the first and only FDA-approved treatment created specifically to treat Demodex blepharitis (DB).1
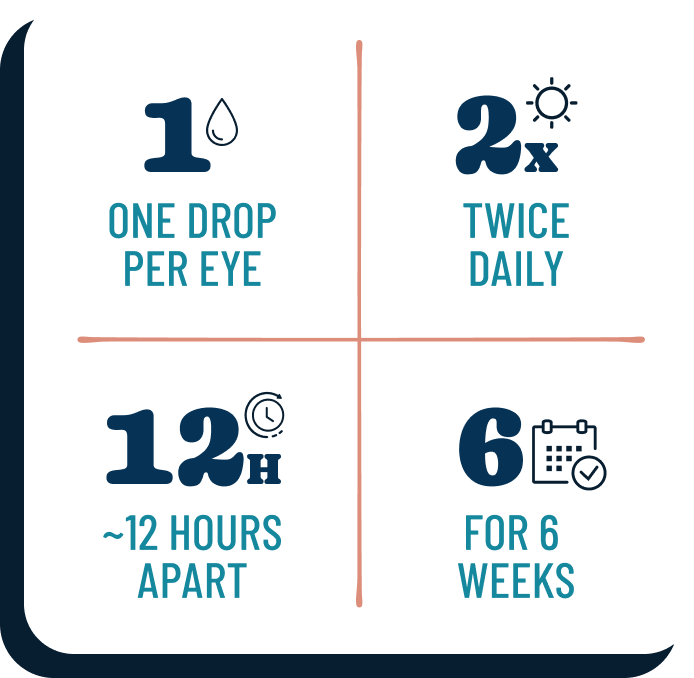
One drop per eye, twice a day, approximately 12 hours apart, in just 6 weeks
In 2 combined studies, 50% of patients had a reduction of crusties (collarettes) and 60% of patients had their Demodex mites completely wiped out.2*
Designed for patient comfort
~90% of patients in both trials reported XDEMVY eye drops
as neutral to very comfortable to use.2†
Stay on top of your treatment
It’s important to use XDEMVY for the full 6 weeks.
Reduce eyelid redness
Patients using XDEMVY for DB may see eyelid redness gone in just 6 weeks.1

Maintain eyelid health
Over time, mites may come back, so it’s important to see your eye doctor regularly. If signs and symptoms such as eyelid redness, itching, or crusties (collarettes) return, make an appointment to visit your eye doctor right away so they can check your eyelids for DB.3-5
*XDEMVY for Demodex blepharitis was evaluated in 2 pivotal trials of 833 patients (415 received XDEMVY).6,7
Trial 1: When taken twice daily in each eye for 6 weeks, XDEMVY reduced collarettes to 2 or less for the upper eyelid in 44% of patients (n=209) at Day 43; XDEMVY eradicated mites (mite density of 0 mites/lash) in 68% of patients (n=212) at Day 43.
Trial 2: When taken twice daily in each eye for 6 weeks, XDEMVY reduced collarettes to 2 or less for the upper eyelid in 55% of patients (n=193) at Day 43; XDEMVY eradicated mites (mite density of 0 mites/lash) in 50% of patients (n=203) at Day 43.
†All visits averaged.2
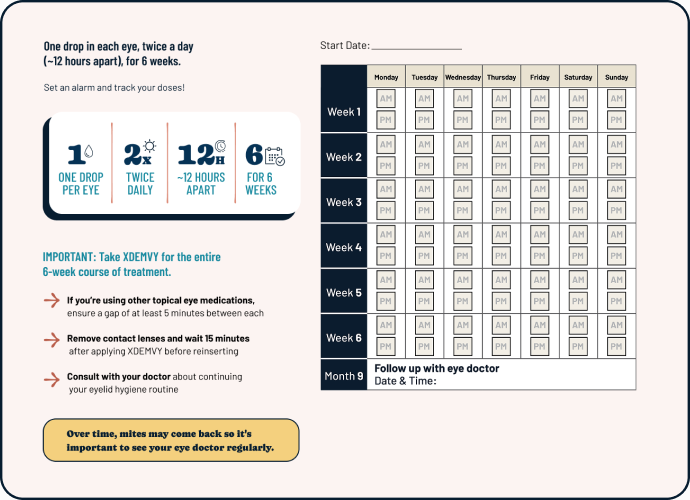
Keep track of your treatment
Use this Dosing Guide to help keep on top of your daily doses of XDEMVY.

IT’S NOT YOU—IT’S DEMODEX MITES
If mites are having a party on your eyelids, it’s time to eliminate the root of the problem.1
Real Results with Xdemvy
View the effect of XDEMVY on Demodex blepharitis after using it twice daily, in each eye, for 6 weeks.
XDEMVY Success Stories
See how others have found relief from Demodex blepharitis with XDEMVY.
References: 1. XDEMVY. Prescribing Information. Tarsus Pharmaceuticals Inc; 2023. 2. Yeu E, Paauw JD, Vollmer P, et al. Safety and efficacy of lotilaner ophthalmic solution (0.25%) in treating Demodex blepharitis: pooled analysis of two pivotal trials. Ophthalmol Ther. 2025;14(3):555-571. 3. Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clin Optom (Auckl). 2018;10:57-63. 4. Sędzikowska A, Osęka M, Grytner-Zięcina B. Ocular symptoms reported by patients infested with Demodex mites. Acta Parasitol. 2016;61(4):808-814. 5. Trattler W, Karpecki P, Rapoport Y, et al. The prevalence of Demodex blepharitis in US eye care clinic patients as determined by collarettes: a pathognomonic sign. Clin Ophthalmol. 2022;16:1153-1164. 6. Gaddie IB, Donnenfeld ED, Karpecki P, et al. Lotilaner ophthalmic solution 0.25% for Demodex blepharitis: randomized, vehicle-controlled, multicenter, phase 3 trial (Saturn-2). Ophthalmology. 2023;130(10):1015-1023. 7. Yeu E, Wirta DL, Karpecki P, Baba SN, Holdbrook M; Saturn I Study Group. Lotilaner ophthalmic solution, 0.25%, for the treatment of Demodex blepharitis: results of a prospective, randomized, vehicle-controlled, double-masked, pivotal trial (Saturn-1). Cornea. 2023;42(4):435-443.


